The biggest news from this past week is probably the announcement that Pfizer’s BioNTech and COVID-10 vaccine is 90% effective in a trial with 43,538 participants (where half received placebos). During the trial, 94 contracted COVID-19 (mostly of whom were in the placebo group, it seems). Pfizer plans to continue the trial until it reaches 164 cases of COVID-19.
In this week’s article, we will be discussing the path forward and what comes next. When can we expect wide-scale control of the pandemic?
The Vaccine
Vaccines work by teaching your immune system to recognize and attack pathogens, and helps to train your immune system to cope with the disease. There are two approaches to making vaccines:
- The traditional approach is to use dead or weakened (attenuated) pathogens that can train your immune system without getting you sick. These vaccines are typically grown in biological systems (either grown in eggs or cell-based)
- The new approach is to use RNA and DNA materials. These are generic materials that constitute only a portion of the genetic code that represents the pathogen. Rather than administer the whole pathogen, this method injects you only with the genetic code that allows your body to be trained to identify and kill the pathogen. This means the vaccine uses less material and can be produced faster.
Pfizer’s vaccine is a type of the second approach. It is an mRNA vaccine that trains the body’s immune system to recognize the coronavirus’ signature “spike proteinâ€. Moderna’s vaccine also uses this approach, so it is likely that the company will announce similar results.
The global pandemic has accelerated the development of this second approach by decades. What is astounding is that not only is this the fastest vaccine in history to be developed, but if approved, will also be the first vaccine made from the RNA approach that will be used on humans.
Where are we in the development cycle?
Before the pandemic, the fastest a vaccine has ever been developed and approved was 5 years (see Figure 1). For COVID-19, the goal is to do so in less than 18 months (give or take 9 months). Even after last week’s announcement, Pfizer’s data collection will still continue (it’s technically still in Phase 3). Before the FDA approves the vaccine for emergency use, an additional two months of follow-up on safety is required.
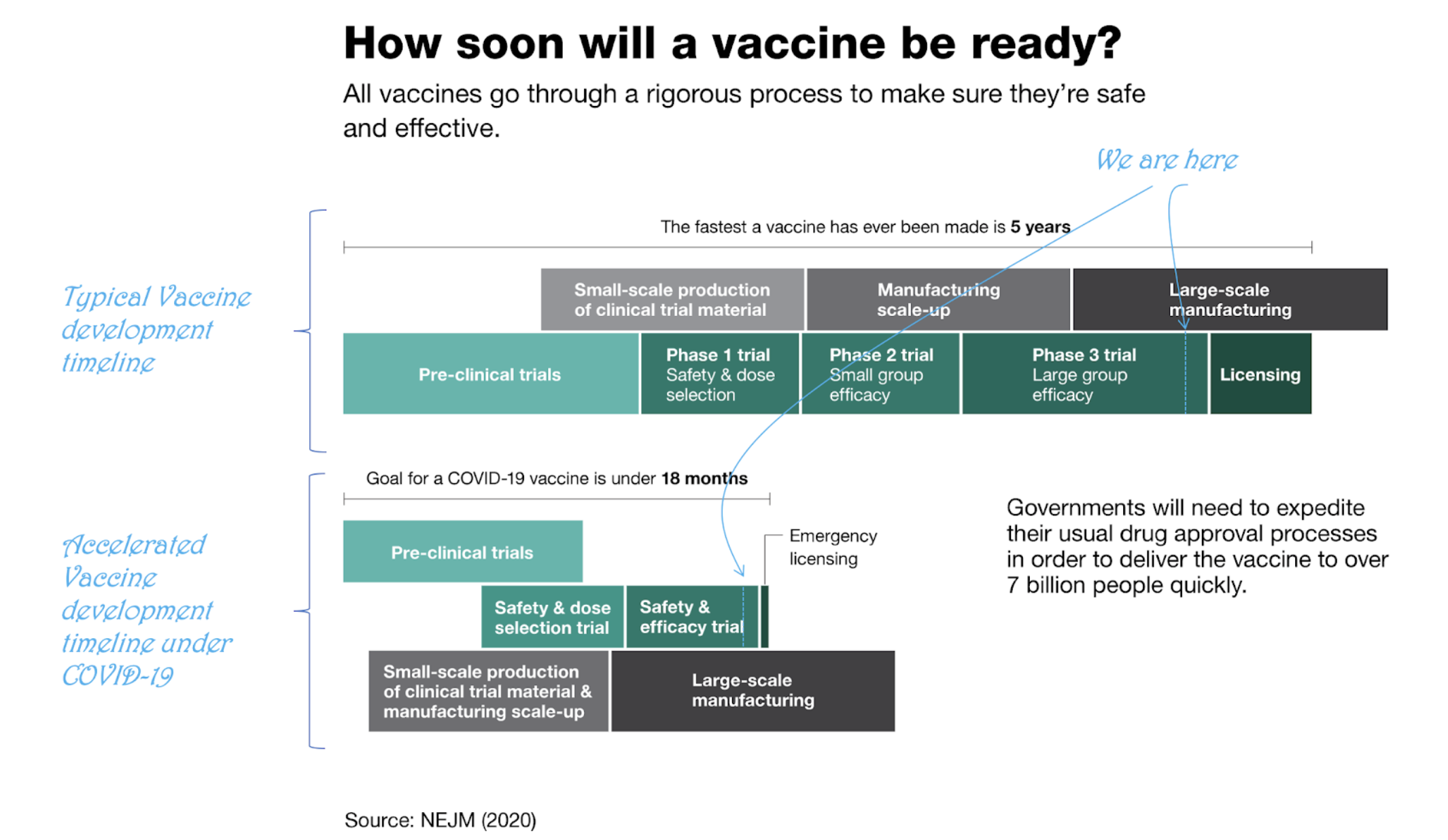
How does a vaccine end a pandemic?
The pandemic will only end if we can reduce the rate at which the disease spreads. You can achieve this through herd immunity. Herd immunity happens when a large segment of a population becomes immune to a disease, making the spread of the disease from person-to-person unlikely. The end result is the protection of the whole population, for both the immune and the nonimmune.
There are two ways you can achieve herd immunity.
- Achieving herd immunity naturally by having enough people get sick and develop antibodies. This approach has not worked well (case in point: Sweden, a country that did not initiate a lockdown and experienced not only a high death rate from the disease but also suffered severe economic downturn).
- Achieving herd immunity via vaccination by having enough people get vaccinated so that the remainder of the population that cannot / will not get the vaccination is protected.
Achieving herd immunity through vaccination is the likely path to ending this pandemic and returning to normalcy. So how do we get the vaccine to 7 billion people?
The challenges ahead
It takes time to immunize the individual
Currently, Pfizer’s vaccine requires 2 doses administered three weeks apart. And only about 28 days after the first 1st dose significant antibodies are reported. In other words, it may take up to a month before the vaccine can immunize the individual.
Logistical challenges to store the vaccine at -80 degrees C
As mentioned, Pfizer’s vaccine is an mRNA vaccine. These types of vaccines, although fast to produce, are highly temperature sensitive. Pfizer’s vaccine will need to be stored at -80 degrees celsius. This means to distribute the vaccine, you have to maintain an uninterrupted cold chain distribution system at this temperature.
Under ideal conditions, this is challenging, even in developed countries. This would be nigh impossible in emerging countries where the infrastructure is not as developed. A recent study by DHL and McKinsey showed that the stringent temperature requirement (of -80 degrees celsius) may prevent large swaths of the global population from gaining access to the vaccine (Figure 2).
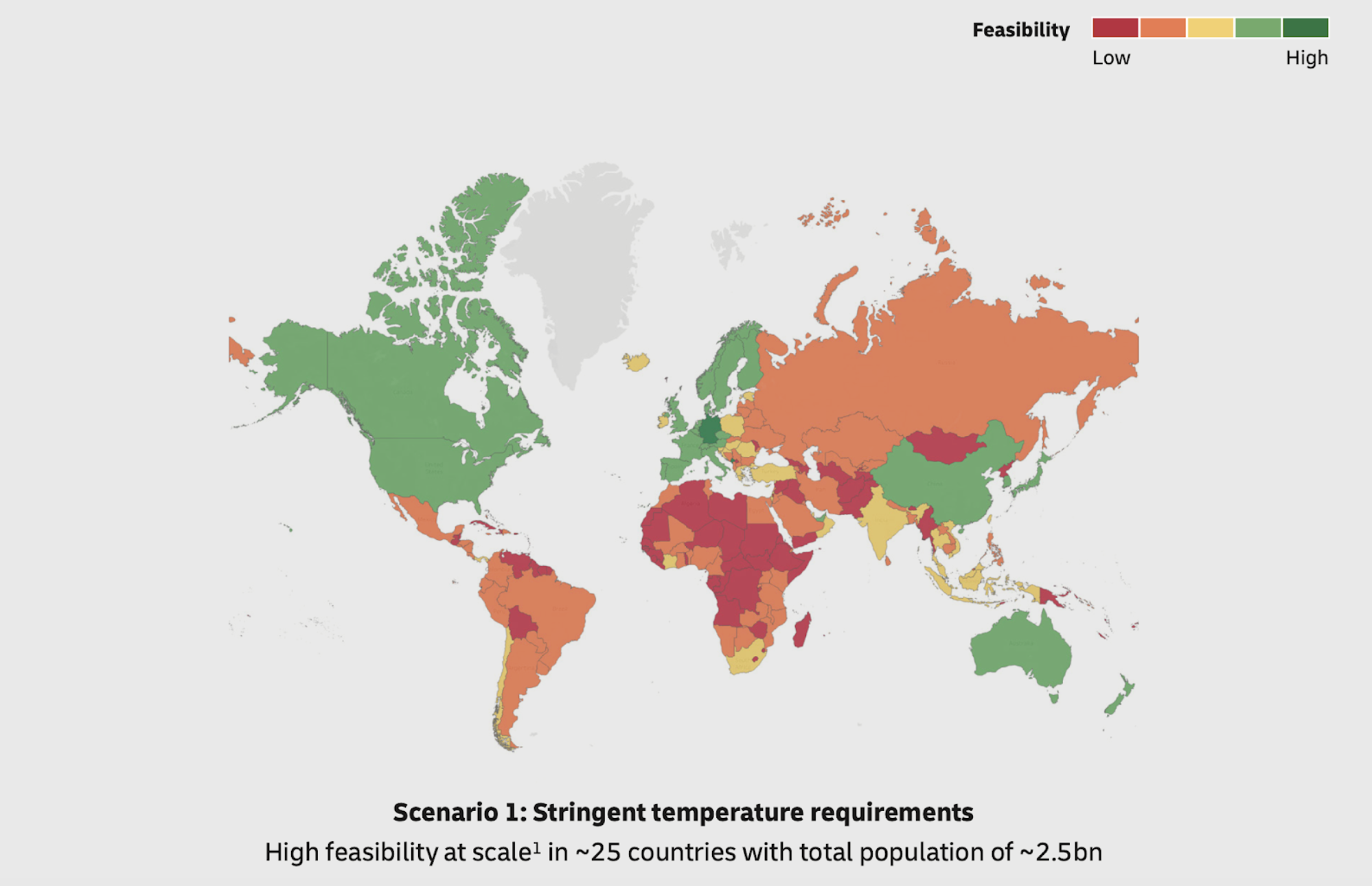
Even in the US, this would pose significant logistical challenges. To maintain -80 degrees celsius, special refrigeration equipment, which many hospitals and clinics in the US do not have, is required. Storage during shipping will require the use of dry ice (frozen carbon-dioxide, which is in short supply due to the decline in ethanol production – of which carbon-dioxide is typically a byproduct of). And finally, at the final distribution location, the vaccine can be stored in a refrigerator for only up to five days.
The good news is that other vaccines in development may not require stringent refrigeration – the downside is that you may have to wait longer for access to these vaccines. Moderna’s may only require -20 degrees celsius, while the vaccine developed by AstraZeneca and Oxford University, must be kept cool but not frozen. At this writing, there are 53 vaccines undergoing clinical trials on humans, and at least 12 are in large scale Phase 3 testing. Pfizer is the first to announce results.
Scaling up production takes time
Pfizer had mentioned that it will produce enough doses to immunize 15 – 20 million people by the end of the year. It is likely that the deployment strategy will be focused on immunizing people with high risk of exposure (medical and essential workers) and vulnerable populations (the elderly).
At the moment, we are unsure of how the vaccines will be distributed. Most countries have secured deals with different drug manufacturers to guarantee supply. But to vaccinate 7 billion people, we may need 14 billion doses (assuming it takes 2 doses to complete the regimen). It may take years for the manufacturing ramp up to achieve this scale.
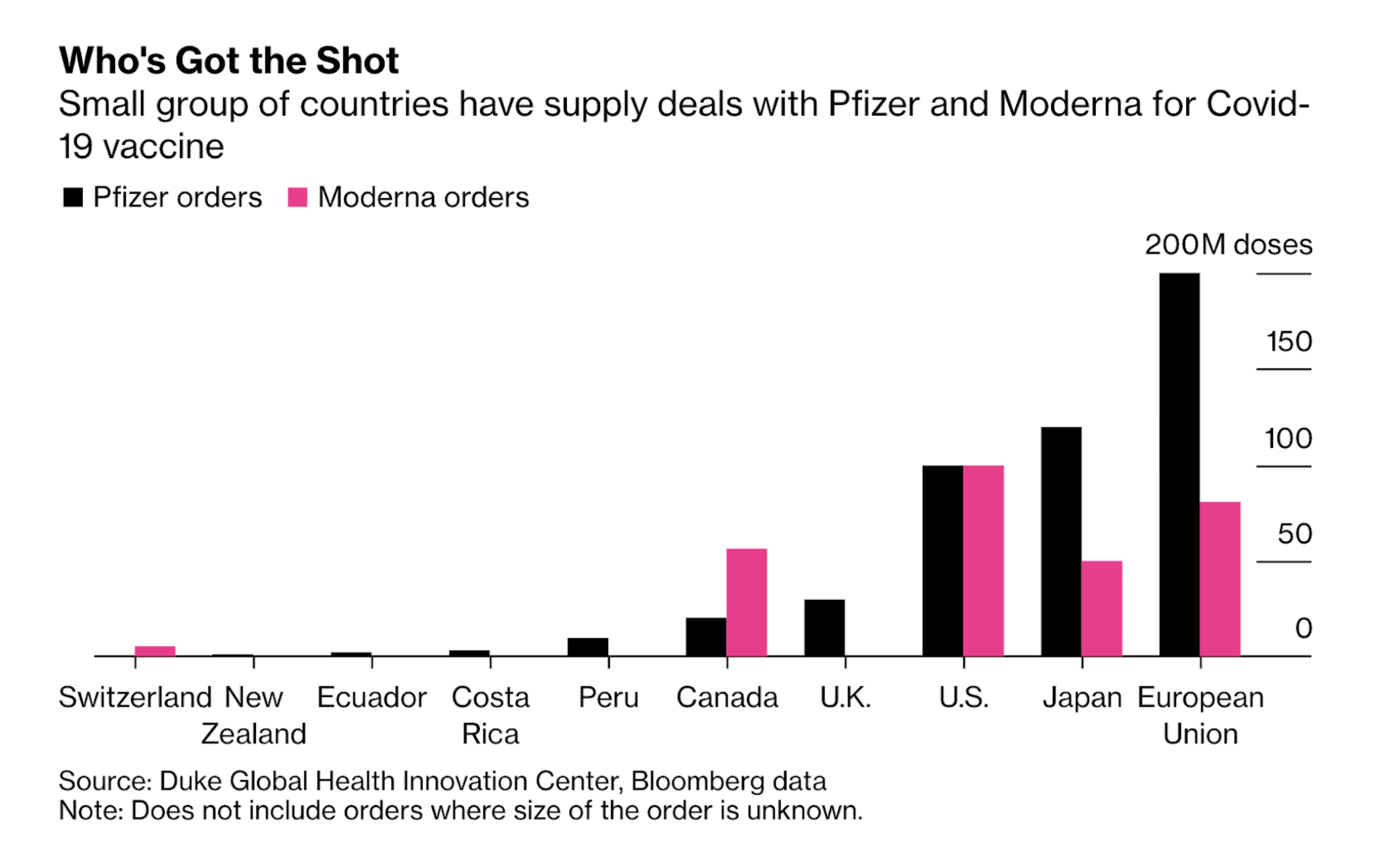
By the end of next year, Pfizer is expected to have the capacity to manufacture about 1.3 billion doses (or vaccinate about 650 million people). Most have been secured by the US, UK, Europe and Japan (about 80% of the supply).
Immunity may not last forever
Studies are still ongoing, but there is evidence that COVID-19 re-infection is possible. What this means from a vaccine development standpoint is unclear. There is a possibility that the population will require periodic re-immunization, similar to the flu vaccine (in the US, people are encouraged to take the flu vaccine every year since the virus changes rapidly).
Achieving herd immunity
As if creating, manufacturing and distributing the vaccine is not difficult enough. To achieve herd immunity, you need a lot of people to accept the immunization. We can estimate the required vaccination level if we know the following two factors:
- The efficacy rate of the vaccine. Pfizer’s currently stands 90%, but there’s a good chance that this number will go down as more people receive the vaccine.
- The duration of protection. Currently, we do not know how long the vaccine will protect the individual from infection.
If we assume an effectiveness rate of 80% for the vaccine, then we must vaccinate about 80 – 100% of the population to achieve herd immunity, every year for the first few years (assuming immunity lasts 1 year). This is an unprecedented level of vaccination coverage. We have never attempted this level of vaccination nationally, let alone globally.
In the US (‘the land of the free’ – where it’s difficult to even mandate mask wearing), the annual flu vaccination rate is about 48% for adults, which is about half the percentage needed to achieve COVID-19 herd immunity.
The road ahead
Ok, so far we’ve outlined all the challenges ahead before we end the pandemic. Nevertheless, the market was optimistic (perhaps overly so?). When the Pfizer news broke, the markets shifted. Shares of companies in the sectors that have struggled initially have jumped, while shares of companies in the tech sector, which gained throughout the pandemic, went down. Still, this shift might be premature.
The world still faces significant challenges in order to produce and distribute the vaccine at a global level. In all likelihood, it will take the better part of 2021 to get the majority of the population vaccinated to significantly slow down the spread. And while we wait, as of this writing, daily cases in the US have surpassed 150,000, with regulators considering a second wave of lockdowns.









